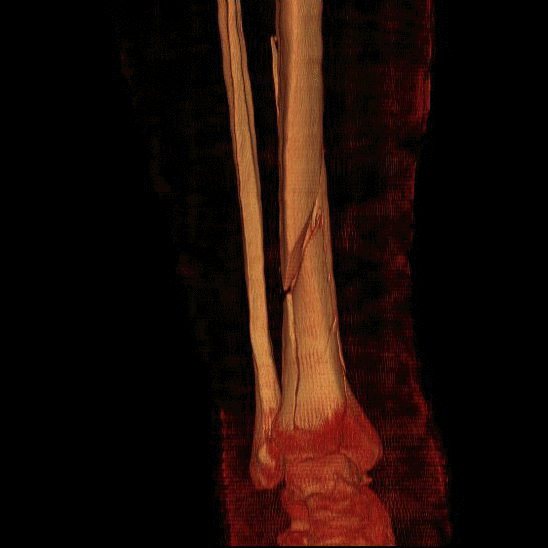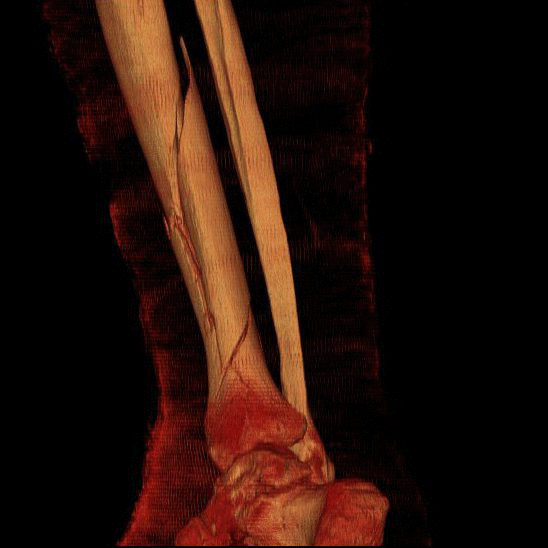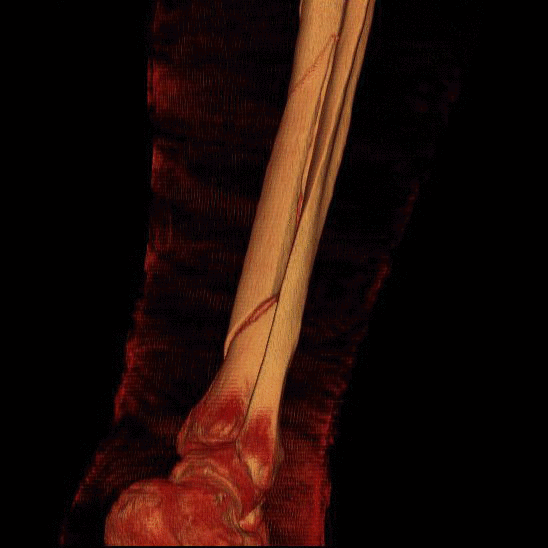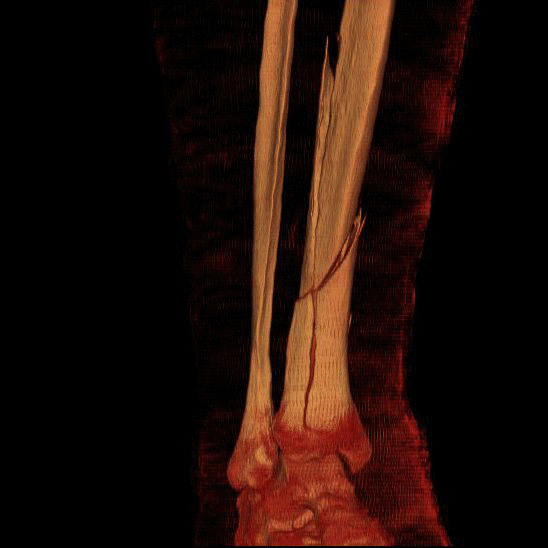
Bone as a Material
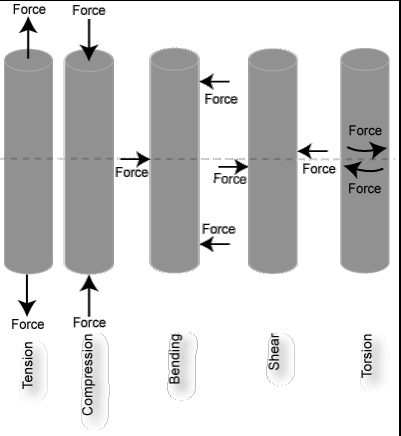
Bone can be examined as a material - and the material properties of the bone can be characterized by the load- deformation relation. By applying a load to a tissue such as bone, then the change in length (deformation) can be measured.
Intrinsic material properties of bone can be expressed in two other measures:
Stress: force per unit area
Strain: change in length relative to original length
Stiffness of the bone is the relationship between stress and strain and this relationship can be plotted like a graph (see figure 2.1). Each region of the slope provides a reflection on the bone’s material properties:
Elastic/linear region: an area of low stiffness, (calculation of this relationship provides a number =elastic modulus). This is the region in which changes in the original length of the material will return to normal length. On figure 2.1 this is the region which is linear.
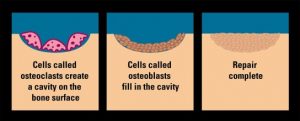
Plastic region: high stiffness region – where the material yields and damage accumulate here due to the rearrangement of internal structures. On figure 2.1, this is the non-linear (curved) region of the graph.
Ultimate yield point - Following the plastic region where the material 'breaks'.
The area under the curve indicates the amount of energy absorbed upon application of force.
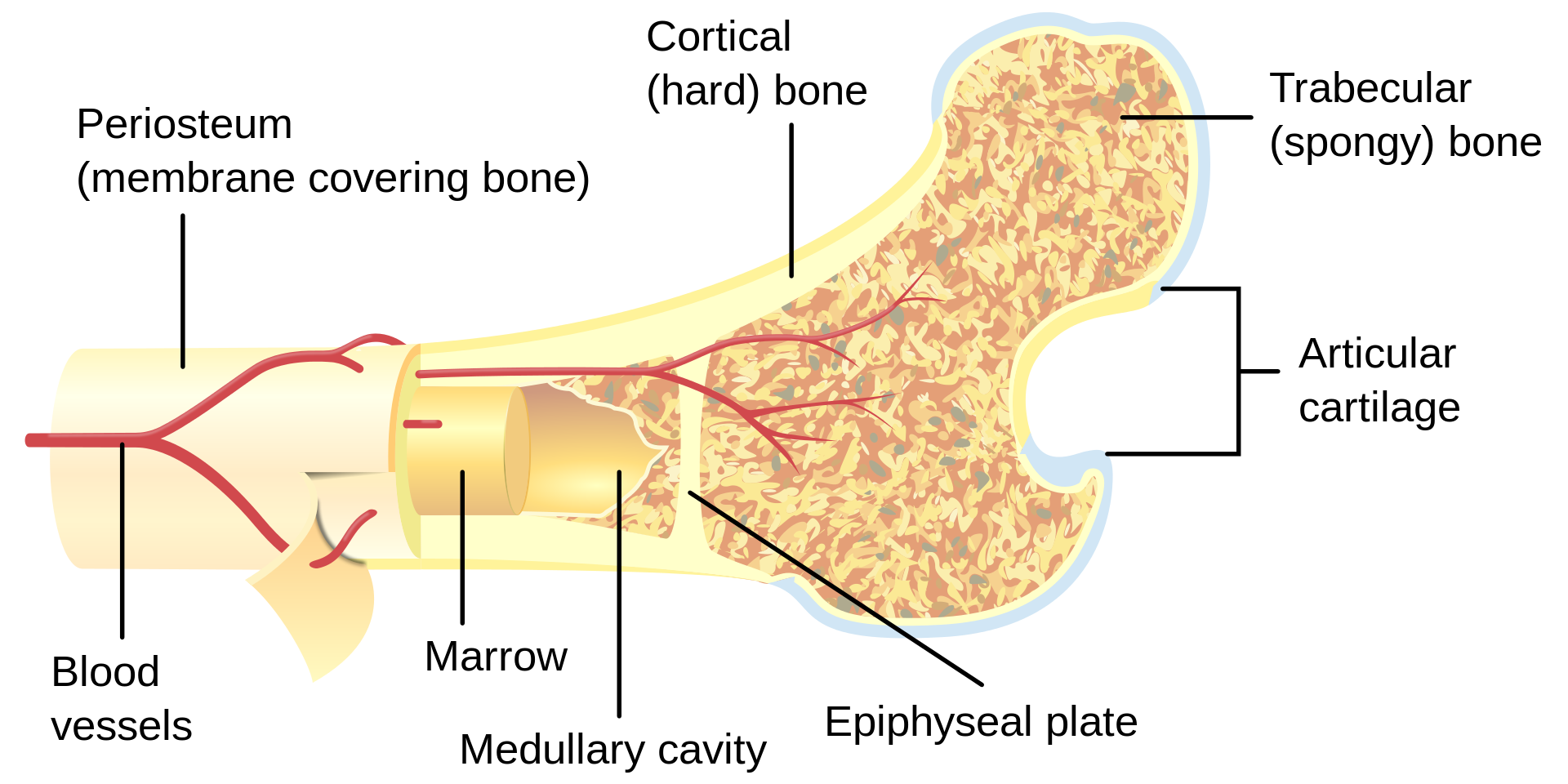
Bone as a structure
Bone can also be characterized by its structure. Unlike the material properties of bone, structural properties are dependent on the geometry of the bone. Size, shape, cross-sectional area, and trabecular orientation in the body (ie., direction that it encounters compressive loads) determine the biomechanical properties. The stress that a bone can withstand depends significantly on the size (cross-sectional area) of that bone and therefore those bones whose function is to withstand large stresses must have a large cross-sectional area -right back to the connection between structure and function!
FACT: most asymptomatic bones can withstand forces between two and five times what they normally are subjected to in activities of daily living.